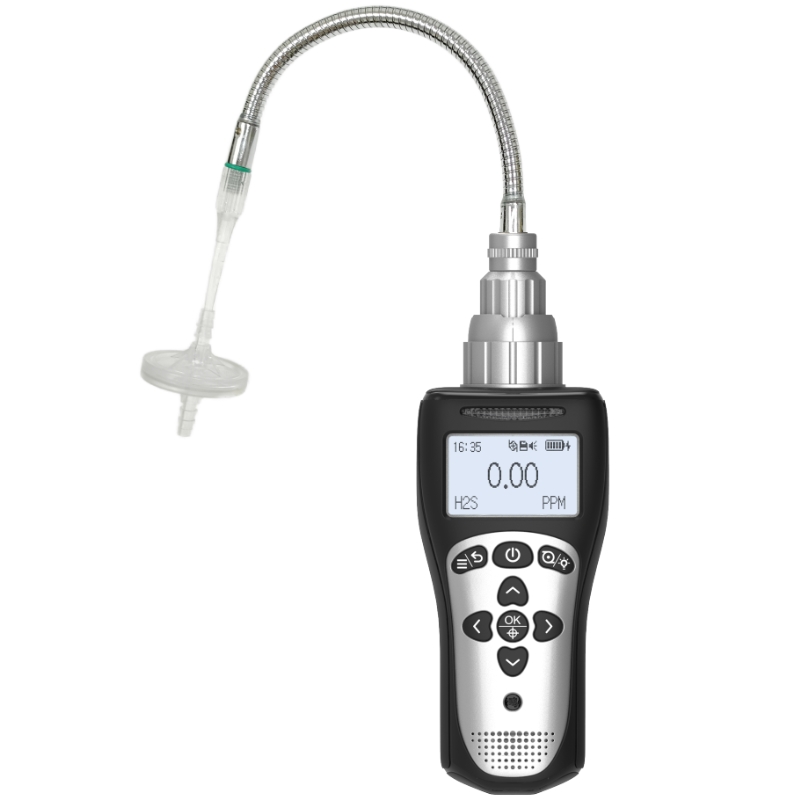
How Does a Carbon Monoxide Detectors Work?
Carbon monoxide is known as the “silent killer” because it is colorless, odorless, and tasteless, yet can rapidly cause death at high concentrations. In industrial safety and commercial buildings, carbon monoxide detectors serve as a critical lifeline. But what exactly happens inside these devices when they flash numbers on a screen or emit a shrill alarm? For overseas users, understanding how carbon monoxide detectors function not only aids in selecting the most suitable tool but also ensures proper usage and maintenance, thereby preventing safety breaches caused by false alarms or equipment failure. Below, Yiyuntian Eranntex delves into this critical technology.

I. Core Mechanism: Chemical Reactions in Electrochemical Sensors
Currently, the vast majority of portable and industrial carbon monoxide detectors on the market utilize electrochemical sensor technology. These sensors are renowned for their high sensitivity, low power consumption, and excellent linear output.
Their operating principle resembles that of a miniature fuel cell. Inside the sensor, there is a working electrode, a counter electrode, and a membrane saturated with electrolyte. When external carbon monoxide gas permeates through the membrane into the sensor, an oxidation reaction occurs on the surface of the working electrode.
Specifically, carbon monoxide molecules lose electrons. These electrons flow through an external circuit to the counter electrode, generating a weak current. The critical point is: the magnitude of this current is directly proportional to the concentration of carbon monoxide in the environment. In other words, higher CO concentrations result in more vigorous chemical reactions and stronger currents. The detector's microprocessor captures this current signal, amplifies it, and converts it through analog-to-digital conversion. The result is displayed on the screen as a PPM (parts per million) reading. When the current value corresponds to a concentration exceeding a preset safety threshold (e.g., 35 PPM or 50 PPM), the device triggers an alarm.
II. Measurement Unit: Understanding PPM
To understand how the detector operates, one must grasp the language it employs—PPM (Parts Per Million).
Unlike “%LEL” which indicates the lower explosive limit, PPM describes extremely low concentrations of toxic gases. 1 PPM signifies one part of carbon monoxide per million parts of air volume. Given the human body's extreme sensitivity to carbon monoxide, prolonged exposure to concentrations as low as several dozen PPM can cause health damage.
Electrochemical sensors are specifically designed to detect these minute changes. They can detect concentrations as low as 1 PPM or even lower, making them ideal for protecting personnel from both chronic and acute poisoning.
III. From Signal to Alarm: Intelligent Processing and Alarm Logic
A simple chemical reaction is insufficient; the detector also requires a “brain” to process the data. Modern carbon monoxide detectors integrate high-performance microprocessors internally, responsible for executing complex logical judgments.
Data Conversion: The microprocessor converts the sensor's analog current signal into a digital signal and performs temperature compensation. This is because temperature variations affect the rate of chemical reactions; the compensation mechanism ensures accurate readings in extreme cold or heat.
Time-Weighted Average (TWA): Carbon monoxide hazards are often cumulative. Smart detectors monitor not only instantaneous concentrations but also calculate average levels over extended periods. For instance, occupational safety standards (like OSHA in the U.S.) stipulate that workers should not exceed an average exposure of 50 PPM over an 8-hour period. Detectors track this data through algorithms to prevent harm from prolonged low-level exposure.
Multi-level Alarms: To differentiate hazard severity, detectors typically feature two alarm levels. A low alarm (e.g., 35 ppm) indicates potential risk requiring ventilation checks; a high alarm (e.g., 100 ppm or 200 ppm) signifies immediate danger necessitating prompt evacuation.
IV. Maintenance and Lifespan: The Consumable Nature of Electrochemical Sensors
Understanding the operating principle is equally vital for recognizing the device's limitations. Electrochemical sensors are consumable components.
As chemical reactions persist, the electrolyte within the sensor gradually depletes or dries out, and electrode performance degrades. This explains why manufacturers typically specify a lifespan of 2 to 5 years for CO sensors. Even when the device is inactive, the sensor undergoes natural aging.
Furthermore, since sensors rely on chemical reactions, certain substances (such as hydrogen sulfide or silicone vapors) can “poison” the electrodes, causing permanent failure. Therefore, regular “calibration”—the process of introducing a known concentration of standard gas to adjust the sensor's readings—is the only way to maintain its accuracy. Neglecting this step may cause the detector to display erroneous safety readings, lulling users into a false sense of security.
In summary, carbon monoxide detectors transform invisible gas threats into visual numerical data through sophisticated electrochemical sensing technology, then assess risks via intelligent logic. They are not merely beeping boxes, but complex systems integrating chemistry, electronics, and software algorithms. For overseas users, understanding this principle fosters greater respect for the device: performing regular bump tests, replacing sensors on schedule, and avoiding exposure to interfering substances. Only by truly understanding how it operates can we optimize its performance, making it the most loyal guardian of our safety.
Related information
-

How to Calibrate an Oxygen Detectors to Ensure Accuracy?
In fields such as industrial safety, confined space operations, environmental monitoring, and even medical support, the measuremen...
2026-01-14 -

What is the measurement range of an oxygen detectors?
Oxygen detectors are indispensable equipment in industrial safety, environmental monitoring, and confined space operations. Accura...
2026-01-13 -

What is the normal oxygen concentration for an oxygen detectors?
Oxygen (O₂) is a vital gas for sustaining human life and most combustion processes. However, both excessively high and low oxygen...
2026-01-12 -

How Do Nitrogen Oxide Detectors Work?
Against a backdrop of growing global concern over air quality and emissions compliance, the precise monitoring of nitrogen oxides ...
2026-01-09 -

Operating Instructions for Nitrogen Oxide Detectors
Nitrogen oxides (NOₓ, primarily comprising nitrogen monoxide NO and nitrogen dioxide NO₂) are significant atmospheric pollutants...
2026-01-07










 info@eranntexgas.com
info@eranntexgas.com


 13480931872
13480931872